As kingdoms go, Protista is a real mess.
The category began with famous 19th-century zoologist Ernst Haeckel, who drew a gnarled tree diagram in 1866 splitting life on Earth into three major branches: Animalia, Plantae, and Protista. Haeckel used the term Protista to describe the microscopic organisms that didn’t look like plants or animals—in ancient Greek, prōtos means “primordial” or “the very first.”
Haeckel pointed out the troublesome task of sorting “all those doubtful organisms of the lowest rank, which display no decided affinities nearer to one side than to the other, or which possess animal and vegetable characters united and mixed.”
A century later, American ecologist Robert Whittaker proposed a new five-kingdom system: animals, plants, fungi, bacteria—and protists. He kept Haeckel’s term to cover the single-celled organisms whose DNA lived inside a nucleus and didn’t fit into the definitions for the other four kingdoms. While the other kingdoms of life are united by features they share in common, protists are grouped together by what they are not.
Protists don’t fit in with bacteria because of their larger cell size and nuclei. That’s more a plant, fungus, and animal thing. But they are excluded from the plant and animal kingdoms because they are not multicellular. Well, some of them are—but if so, they don’t have specialized tissue types, like animals and plants do. While plants make their own food from sunlight, animals eat other things to live, and fungi are decomposers, there are protists that do each of those things. Some protists are even capable of both eating other creatures and photosynthesizing.
Allow me to introduce you to some of these often overlooked oddball organisms––some bizarre, some slimy, and some essential to life on Earth.
Giant kelp: A giant protist

Despite looking very much like a plant, kelp and other seaweeds are considered protists.
Plants use vascular systems to ferry water and nutrients within themselves, and they have different kinds of tissues made from specialized cells, like roots, bark, or leaves. Kelp doesn’t have any of that fancy stuff. Instead, it’s made of the exact same tissue, from its clinging holdfasts at the ocean’s floor to its frond-like tips. Each individual kelp cell pumps fluids and nutrients for itself.
While they don’t exactly qualify as a plant, kelp and other seaweeds are sometimes referred to as “plant-like” protists. Kelp belongs in the brown algae class, along with its seaweed cousins. Kelp’s closest genetic relatives, however, are not only single-celled but incredibly tiny—less than 10 micrometers across. Giant kelp, meanwhile, is the largest protist in the world.
These undulating giants are among the few multicellular protists, and perhaps the most appreciated by humans as well as the many creatures that call their undersea forests home.

Miamiensis avidus, eater of shark brains

Most protists are harmless, and some are helpful. But the ones that make headlines are those that cause visible havoc. Like when a massive algal bloom of Heterosigma akashiwo killed fish all over San Francisco Bay in August 2022. Or the time, in 2017, when a mysterious protist began devouring sharks’ brains.
That year, an estimated 1,000 dead leopard sharks were stranded on the shores of the San Francisco Bay, their cause of death unknown. UC San Francisco graduate student Hanna Retallack went out with a giant hook to drag the dead sharks on shore. Back at the lab, Retallack peered at their brain tissue and found round, hairy blotches nestled inside spiral-shaped burrows, as if parasites had drilled in as they ate.
“Their brains were all exploded,” says Joe DeRisi, Retallack’s advisor and a UCSF biochemist whose lab identifies mysterious brain infections.
Gene sequencing revealed them to be the protist Miamiensis avidus, a kind of ciliate. Ciliates are translucent protists with a halo of hair-like appendages called cilia, which they use to scoot around in the water like tiny motorboats as they hoover up microscopic prey. According to DeRisi, M. avidus is no zombie, actively seeking out brains—it’s just an opportunist. When heavy rains dump fresh water into Bay estuaries, the waterways become less salty. That may weaken sharks’ immune systems, expose them to toxins, or make Bay water more M. avidus-friendly. The ciliates venture into Bay silt, where unlucky sharks roaming the mudflats sniff them up.
From the nose, DeRisi says, “they take the highway right to the brain.”
Brainless but not untalented: Slime molds

For decades, slime molds were thought to be fungi. But recently, these infamously odd amoebas have oozed their way into the kingdom Protista.
Slime molds are sometimes called “fungus-like” protists. They reproduce by making fruiting bodies with spores like fungi do, and play a similar ecological role––releasing nutrients back into the cycle of life. But unlike most fungi, slime molds do not build themselves out of chitin, and genetic analyses now place them in an evolutionary category all their own.
Slime molds in the Myxomycetes family spend most of their life cycle living and feeding as individual amoebas. But when food is scarce, they can merge to become a giant single cell, sometimes as big as two feet across, containing all the formerly independent nuclei inside it. This blob oozes through its environment, stretching in different directions to form protrusions called pseudopods, Greek for “false feet.” The blob pulses toward signs of bacterial feasts, which they will surround and then engulf.

“There have been reports of people seeing larger plasmodia pulsating and moving over plants and thinking they were some form of extraterrestrial life,” says Diana Wrigley de Basanta, biologist and contributor to the slime mold research project Myxotropic.
Slime molds are all around us. They’ve been found in the driest deserts, snow and ice, bogs, treetops, inside cacti, and everywhere researchers have looked. In the Bay Area, commonly spotted slime molds include dog vomit slime mold (Fuligo septica) and wolf’s milk (Lycogala epidendrum). To glimpse these ethereal slimes, you’ll have to slow down and look carefully.
Living in a glass house: Diatoms
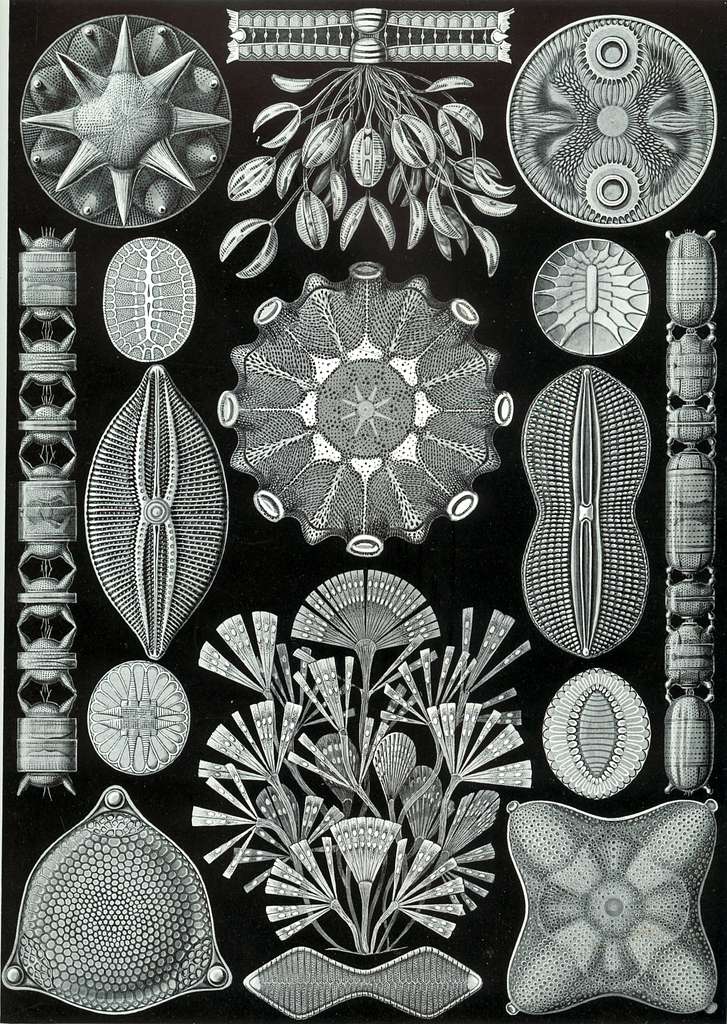
A drop of Bay water contains hundreds of thousands of protists. Among these microscopic drifters are algae, which are responsible for half the photosynthesis on Earth, according to Kris Niyogi, a UC Berkeley and Lawrence Berkeley National Lab algae researcher.
“They’re essential for driving the geochemical cycles that sustain life on Earth,” says David Booth, another protist researcher who is an evolutionary biologist at UC San Francisco. “Protists, especially in the oceans, provide over half of the world’s oxygen, fix a quarter of its carbon, and help recycle essential nutrients.”
One such group of sunshine-guzzling ocean algae are the diatoms. The word algae may bring to mind squishy, mushy green gunk in a pond. But diatoms are far from mushy: they form elaborate crystalline shells out of biological glass, made from silicon they collect from the water. Why diatoms build such striking shells is unknown. (The diatomaceous earth used in gardening is made from the fossilized shells of these protists.)

These oxygen-producing powerhouses also provide clues to understanding eons past. When a diatom dies, its glass house sinks to the bottom of the ocean. Layers of fossilized protist shells give paleontologists a snapshot of populations at different geologic times. Changes in the dominant shell shapes can help researchers reconstruct what the world’s climate was like long ago.
Salt-pond watercolors: Dunaliella salina

You may see them while crossing the Dumbarton Bridge, or from a plane landing nearby: a patchwork of pink, carrot-orange, green and purple geometric shapes, fringing the southern end of San Francisco Bay. These are salt evaporation ponds, remnants of the South Bay’s once-booming salt industry. The reason for their color? Protists (along with other microbes).
Meet the halophiles, Greek for ‘salt loving.’ Salt-tolerant black fungi, pink archaea, purple bacteria, brine shrimp and colorless amoebas all manage to get by in the salt ponds.
The color of a pond can tell you what kind of organism dominates it, says halophile researcher Juergen Polle, a biology professor at the City University of New York and chief scientist at MicroBio Engineering, an algae-pond consulting company. “That is often driven by how much salt is in there,” he says.
Some of the most remarkable colors in the South Bay are a product of a protist known as Dunaliella salina, an oval-shaped green alga with two flailing tails. D. salina thrives in seawater, but it can also survive in ponds with salt concentrations as high as 40 percent. It’s responsible for a spectrum of hues from vibrant green to orange to deep red, thanks to the green pigment it uses to photosynthesize and to the beta carotene it produces when stressed. The saltier the water, the more beta carotene it makes.
“The more Dunaliella salina you have, the more orange it will be,” says Polle.
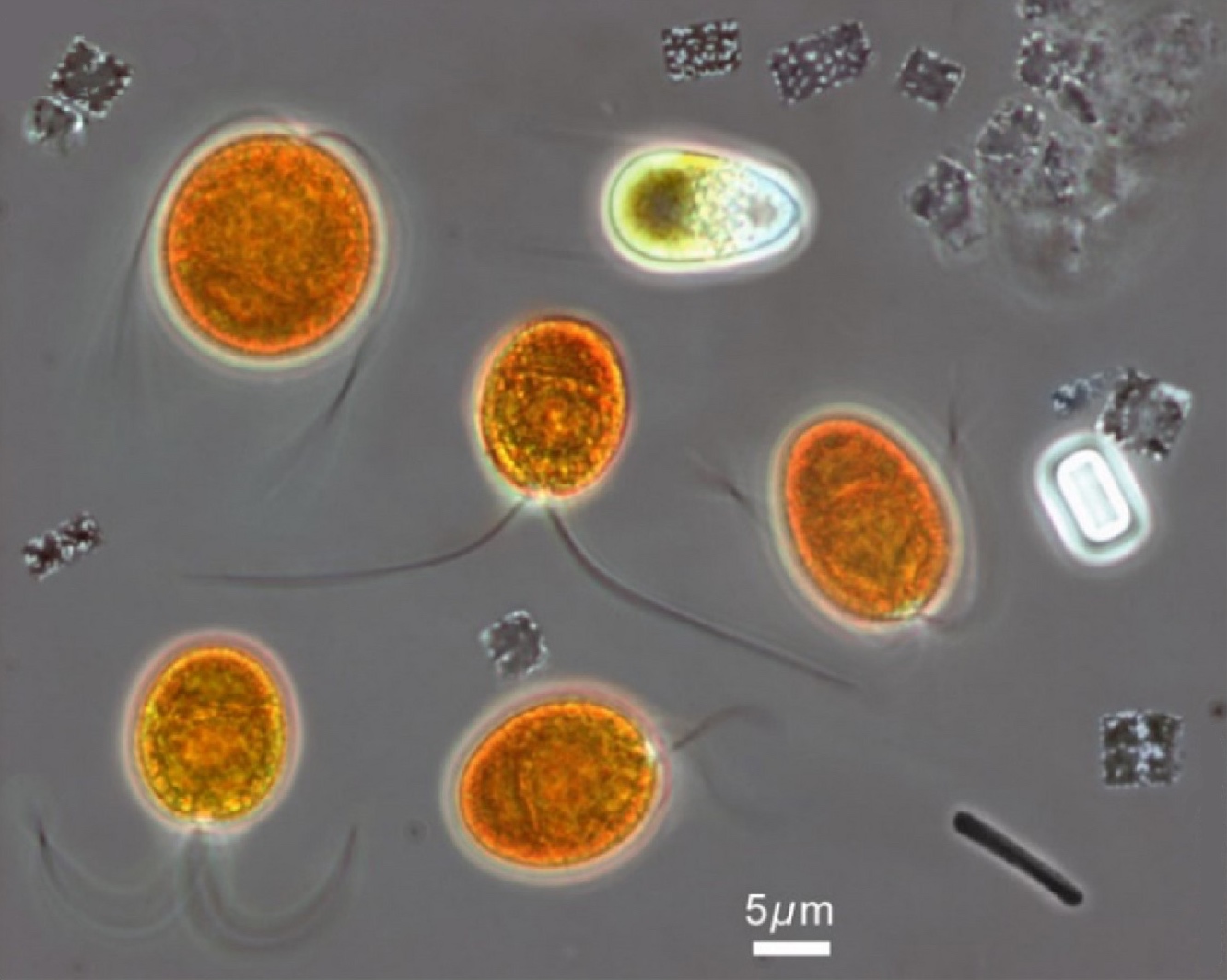
How exactly beta carotene benefits D. salina in these conditions is not entirely clear. According to UC Berkeley’s Niyogi, the stress from living with high salinity affects the protist’s ability to photosynthesize. So, he says, maybe the beta carotene helps shield it from excess sunlight that would damage its cellular machinery.
For over a century, the salt extraction industry dominated the South Bay. But these days, the South Bay Restoration Project has been working to convert former salt ponds back into tidal marsh by breaching levees and unleashing the tides back into these liminal areas.
Seawater is salty, but not salt-pond salty. When Bay water rushes into a salt pond, it upends the ponds’ bespoke microbial ecosystem. Dunaliella salina is freed from its salty confinement, and perhaps can relax a little from pumping out all that beta carotene. As salinity decreases, the vibrant oranges, reds and pinks gradually settle back into greens, blues and browns.

Our closest single-celled relatives: Choanoflagellates
Now and then, UC San Francisco evolutionary biologists clamber down the slippery rocks at Crane Cove Park in San Francisco, with their eyes on the slime. They are sampling the Bay for protists known as choanoflagellates, single-celled predators that may provide clues to the origins of animals.
“They are a window into our own evolutionary history,” says Booth.
Choanoflagellates are single-celled––until they aren’t. An individual choanoflagellate is round and about yeast-cell-sized, with a pleated, funnel-shaped collar that encircles a long, twirling whiptail (hence their name: “choano” meaning funnel, and “flagellate” for the whip-like tail).
But in response to certain conditions, individuals can come together to form elaborate multicellular colonies. Booth’s team is keen to understand how our choanoflagellate-like common ancestors evolved the multicellular tricks that led (eventually) to the complex animals we know and are today.

Choanoflagellate colonies can form a variety of shapes that give them special collective abilities. Some colonies form in a way that helps them swim faster together. Or they settle on an underwater surface and wave their tails in a rhythm that sucks in particles of food better than any individual could. These feats of microscopic teamwork have fascinated scientists like Booth.
“One drop of water in the bay actually represents a really, really broad diversity of evolutionary innovations and evolutionary time,” Booth says.
A crumbling kingdom
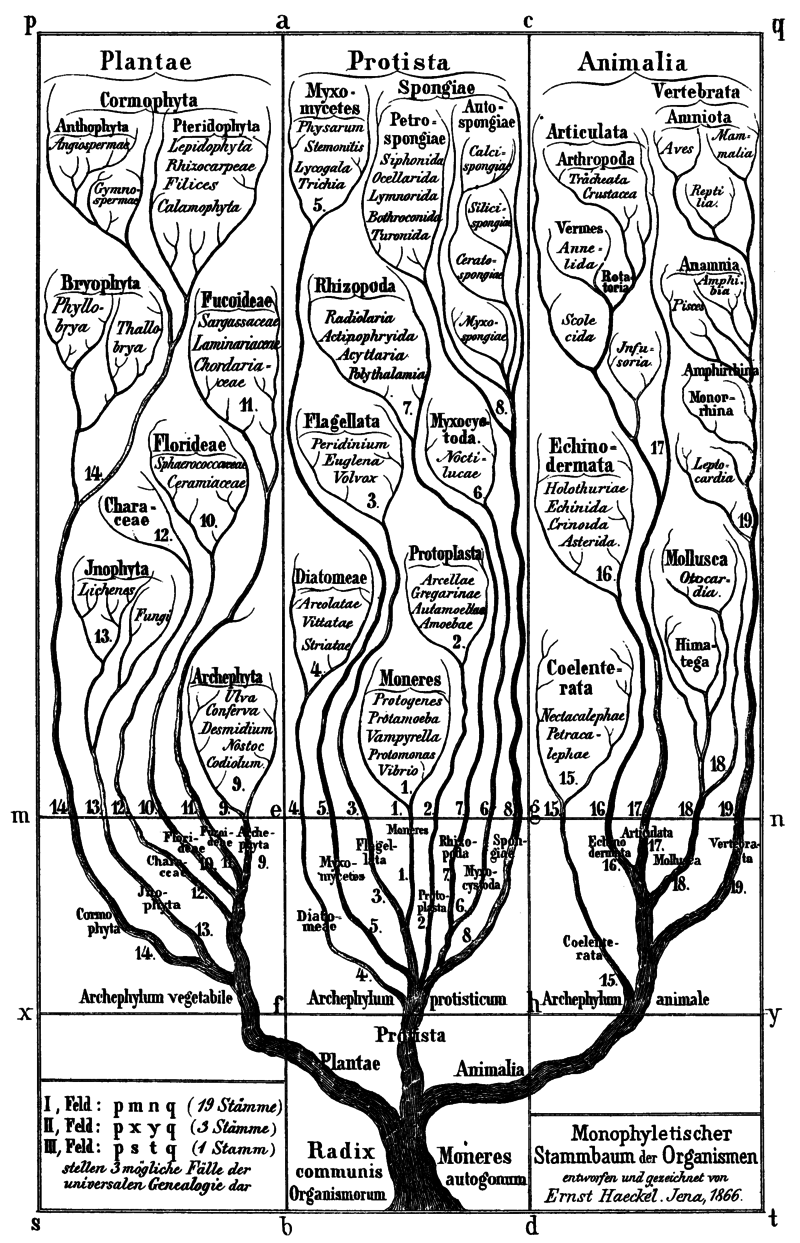
As genetic sequencing has revealed deeper evolutionary ties, the kingdom Protista has been undergoing an overhaul.
“The modern view is that they are not their own kingdom,” says Niyogi, the UC Berkeley algae researcher.
These days, biology students learn about new classification systems, such as the three-domain system (which contains six kingdoms). These are still works in progress.
As for the protists, creating new labels based on what they have in common, instead of defining them by what they are not, is tricky business. Even if Protista no longer reigns as its own kingdom, “protists” might still be useful as an umbrella term for single-celled organisms that keep their DNA inside a nucleus, according to Dave Caron, a marine microbial ecologist at the University of Southern California.
The protists, of course, do not care how humans choose to name or sort them. Caron appreciates them because they are key to understanding our world, and because they are among nature’s marvels.
“They are just unbelievably beautiful,” Caron says. “The vast majority of them are beneficial, and they need to be there.”
Correction: this story has been updated to correct the spelling of Hanna Retallack’s name.



-300x221.jpg)

